Optical and Infrared Spectroscopy
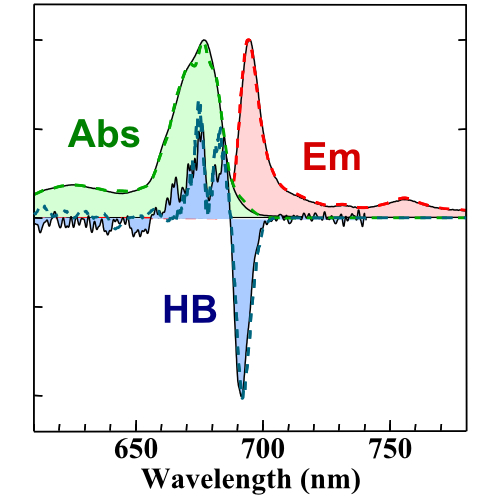
Our group uses optical and infrared (IR) spectroscopy to probe both the atomistic and energetic structure of proteins and pigment/protein complexes (PPCs). Room-temperature absorption and fluorescence spectroscopy provide insight into the optical properties of photosynthetic systems under physiological conditions, while low-temperature hole burning (HB) spectroscopy and fluorescence line narrowing (FLN) spectroscopy offer detailed insight into the interactions between pigments and their protein environment. Isotope-edited IR absorption spectra are used to monitor both both global protein folds (e.g. helix vs. sheet) and local conformational changes (e.g. hydrogen bond formation).
Although spectroscopic measurements provide critical insight into structure and dynamics, it can be extremely difficult to translate raw experimental data into a reliable, functionally-relevant interpretation. Our group specializes in developing quantitative tools for analyzing experimental data, through a combination of experimental standard development and quantum/classical dynamical modeling. Follow the links to learn more about these research areas or scroll down for publications related to spectroscopy.
Selected Publications
- Dutta, Rajesh; Reppert, Mike; "Quantum and classical effects in system-bath correlations and optical line shapes". Phys. Rev. A, 111, 022210 (2025).
- Dutta, Rajesh; Ma, Zifan; Fournier, Joseph A.; Reppert, Mike; "Nonlinear response from linear oscillators: Gas phase 2D action spectroscopy". The Journal of Chemical Physics, 163, 054113 (2025).
- Wat, Jacob H.; Pizzala, Nicolas J.; Reppert, Mike; "Isotope Reverse-Labeled Infrared Spectroscopy as a Probe of In-Cell Protein Structure". The Journal of Physical Chemistry B, 128, 9923-9934 (2024).
- Grechishnikova, Galina; Wat, Jacob H.; de Cordoba, Nicolas; Miyake, Ethan; Phadkule, Amala; Srivastava, Amit; Savikhin, Sergei; Slipchenko, Lyudmila; Huang, Libai; Reppert, Mike; "Controlling Vibronic Coupling in Chlorophyll Proteins: The Effects of Excitonic Delocalization and Vibrational Localization". The Journal of Physical Chemistry Letters, 15, 9456-9465 (2024).
- Ennist, Nathan M.; Wang, Shunzhi; Kennedy, Madison A.; Curti, Mariano; Sutherland, George A.; Vasilev, Cvetelin; Redler, Rachel L.; Maffeis, Valentin; Shareef, Saeed; Sica, Anthony V.; Hua, Ash Sueh; Deshmukh, Arundhati P.; Moyer, Adam P.; Hicks, Derrick R.; Swartz, Avi Z.; Cacho, Ralph A.; Novy, Nathan; Bera, Asim K.; Kang, Alex; Sankaran, Banumathi; Johnson, Matthew P.; Phadkule, Amala; Reppert, Mike; Ekiert, Damian; Bhabha, Gira; Stewart, Lance; Caram, Justin R.; Stoddard, Barry L.; Romero, Elisabet; Hunter, C. Neil; Baker, David; "De novo design of proteins housing excitonically coupled chlorophyll special pairs". Nature Chemical Biology, (2024).
- Ahad, S.; Lin, C.; Reppert, M.; "PigmentHunter: A point-and-click application for automated chlorophyll-protein simulations". The Journal of Chemical Physics, 160, 154111 (2024).
- Lin, Chientzu; Mazor, Yuval; Reppert, Mike; "Feeling the Strain: Quantifying Ligand Deformation in Photosynthesis". The Journal of Physical Chemistry B, 128, 2266-2280 (2024).
- Reppert, Mike; Dutta, Rajesh; Slipchenko, Lyudmila; "The interplay of excitonic delocalization and vibrational localization in optical lineshapes: A variational polaron approach". The Journal of Chemical Physics, 161, 154109 (2024).
- Reppert, Mike; Reppert, Deborah; "Equivalence of quantum and classical third order response for weakly anharmonic coupled oscillators". The Journal of Chemical Physics, 158, 114114 (2023).
- Reppert, Mike; "Bioexcitons by Design: How Do We Get There?". The Journal of Physical Chemistry B, 127, 1872-1879 (2023).
- Dobson, Zachary; Ahad, Safa; Vanlandingham, Jackson; Toporik, Hila; Vaughn, Natalie; Vaughn, Michael; Williams, Dewight; Reppert, Michael; Fromme, Petra; Mazor, Yuval; "The structure of photosystem I from a high-light-tolerant cyanobacteria". eLife, 10, e67518 (2021).
- Chelius, Kevin; Wat, Jacob H.; Phadkule, Amala; Reppert, Mike; "Distinct electrostatic frequency tuning rates for amide I and amide I′ vibrations". J. Chem. Phys., 155, 195101 (2021).
- Baiz, Carlos R.; Błasiak, Bartosz; Bredenbeck, Jens; Cho, Minhaeng; Choi, Jun-Ho; Corcelli, Steven A.; Dijkstra, Arend G.; Feng, Chi-Jui; Garrett-Roe, Sean; Ge, Nien-Hui; Hanson-Heine, Magnus W. D.; Hirst, Jonathan D.; Jansen, Thomas L. C.; Kwac, Kijeong; Kubarych, Kevin J.; Londergan, Casey H.; Maekawa, Hiroaki; Reppert, Mike; Saito, Shinji; Roy, Santanu; Skinner, James L.; Stock, Gerhard; Straub, John E.; Thielges, Megan C.; Tominaga, Keisuke; Tokmakoff, Andrei; Torii, Hajime; Wang, Lu; Webb, Lauren J.; Zanni, Martin T.; "Vibrational Spectroscopic Map, Vibrational Spectroscopy, and Intermolecular Interaction". Chemical Reviews, 120, 7152-7218 (2020).
- Reppert, Mike; Roy, Anish R.; Tempkin, Jeremy O. B.; Dinner, Aaron R.; Tokmakoff, Andrei; "Refining Disordered Peptide Ensembles with Computational Amide I Spectroscopy: Application to Elastin-Like Peptides". J. Phys. Chem. B, 120, 11395-11404 (2016).
- Reppert, Mike; Tokmakoff, Andrei; "Computational Amide I 2D IR Spectroscopy as a Probe of Protein Structure and Dynamics". Ann. Rev. Phys. Chem., 67, 359-386 (2016).
- Reppert, Mike; Roy, Anish R.; Tokmakoff, Andrei; "Isotope-enriched protein standards for computational amide I spectroscopy". J. Chem. Phys., 142, 125104 (2015).
- Reppert, Mike; Tokmakoff, Andrei; "Communication: Quantitative multi-site frequency maps for amide I vibrational spectroscopy". J. Chem. Phys., 143, 061102 (2015).
- Reppert, Mike; Kell, Adam; Pruitt, Thomas; Jankowiak, Ryszard; "Comments on the optical lineshape function: Application to transient hole-burned spectra of bacterial reaction centers". J. Chem. Phys., 142, 094111 (2015).
- Vrandecic, Kamarniso; Ratsep, Margus; Wilk, Laura; Rusevich, Leonid; Golub, Maksym; Reppert, Mike; Irrgang, Klaus-Dieter; Kuhlbrandt, Werner; Pieper, Jorg; "Protein Dynamics Tunes Excited State Positions in Light-Harvesting Complex II". J. Phys. Chem. B, 119, 3920-3930 (2015).
- De Marco, Luigi; Thamer, Martin; Reppert, Mike; Tokmakoff, Andrei; "Direct observation of intermolecular interactions mediated by hydrogen bonding". J. Chem. Phys., 141, 034502 (2014).
- Lin, Chen; Reppert, Mike; Feng, Ximao; Jankowiak, Ryszard; "Modeling of fluorescence line-narrowed spectra in weakly coupled dimers in the presence of excitation energy transfer". J. Chem. Phys., 141, 035101 (2014).
- Baiz, Carlos; Reppert, Mike; Tokmakoff, Andrei"Ultrafast Infrared Vibrational Spectroscopy In Ultrafast Infrared Vibrational Spectroscopy , M.D. Fayer, editor, Chapter 12, 361-404. Taylor \& Francis, Boca Raton(2013).
- Kell, Adam; Feng, Ximao; Reppert, Mike; Jankowiak, Ryszard; "On the Shape of the Phonon Spectral Density in Photosynthetic Complexes". J. Phys. Chem. B, 117, 7317-7323 (2013).
- Baiz, Carlos R.; Reppert, Mike; Tokmakoff, Andrei; "Amide I Two-Dimensional Infrared Spectroscopy: Methods for Visualizing the Vibrational Structure of Large Proteins". J. Phys. Chem. A, 117, 5955-5961 (2013).
- Reppert, Mike; Tokmakoff, Andrei; "Electrostatic frequency shifts in amide I vibrational spectra: Direct parameterization against experiment". J. Chem. Phys., 138, 134116 (2013).
- Acharya, Khem; Zazubovich, Valter; Reppert, Mike; Jankowiak, Ryszard; "Primary Electron Donor(s) in Isolated Reaction Center of Photosystem II from Chlamydomonas reinhardtii". J. Phys. Chem. B, 116, 4860-4870 (2012).
- Baiz, Carlos R.; Peng, Chunte Sam; Reppert, Mike E.; Jones, Kevin C.; Tokmakoff, Andrei; "Coherent two-dimensional infrared spectroscopy: Quantitative analysis of protein secondary structure in solution". Analyst, 137, 1793-1799 (2012).
- Lessing, Joshua; Roy, Santanu; Reppert, Mike; Baer, Marcel; Marx, Dominik; Jansen, Thomas La Cour; Knoester, Jasper; Tokmakoff, Andrei; "Identifying Residual Structure in Intrinsically Disordered Systems: A 2D IR Spectroscopic Study of the GVGXPGVG Peptide". J. Am. Chem. Soc., 134, 5032-5035 (2012).
- Neupane, Bhanu; Jaschke, Paul; Saer, Rafael; Beatty, J. Thomas; Reppert, Mike; Jankowiak, Ryszard; "Electron Transfer in Rhodobacter sphaeroides Reaction Centers Containing Zn-Bacteriochlorophylls: A Hole-Burning Study". J. Phys. Chem. B, 116, 3457-3466 (2012).
- Jankowiak, Ryszard; Reppert, Mike; Zazubovich, Valter; Pieper, Jorg; Reinot, Tonu; "Site Selective and Single Complex Laser-Based Spectroscopies: A Window on Excited State Electronic Structure, Excitation Energy Transfer, and Electron-Phonon Coupling of Selected Photosynthetic Complexes". Chem. Rev., 111, 4546-4598 (2011).
- Reppert, Mike; "Modeling of Resonant Hole-Burning Spectra in Excitonically Coupled Systems: The Effects of Energy-Transfer Broadening". J. Phys. Chem. Lett., 2, 2716-2721 (2011).
- Reppert, Mike; Acharya, Khem; Neupane, Bhanu; Jankowiak, Ryszard; "Lowest Electronic States of the CP47 Antenna Protein Complex of Photosystem II: Simulation of Optical Spectra and Revised Structural Assignments". J. Phys. Chem. B, 114, 11884-11898 (2010).
- Reppert, Mike; Naibo, Virginia; Jankowiak, Ryszard; "Accurate modeling of fluorescence line narrowing difference spectra: Direct measurement of the single-site fluorescence spectrum". J. Chem. Phys., 133, 014506 (2010).
- Acharya, K.; Neupane, B.; Reppert, M.; Feng, X.; Jankowiak, R.; "On the Unusual Temperature-Dependent Emission of the CP47 Antenna Protein Complex of Photosystem II". J. Phys. Chem. Lett., 1, 2310-2315 (2010).
- Neupane, Bhanu; Dang, Nhan C.; Acharya, Khem; Reppert, Mike; Zazubovich, Valter; Picorel, Rafael; Seibert, Michael; Jankowiak, Ryszard; "Insight into the Electronic Structure of the CP47 Antenna Protein Complex of Photosystem II: Hole Burning and Fluorescence Study". J. Am. Chem. Soc., 132, 4214-4229 (2010).
- Reppert, Mike; Naibo, Virginia; Jankowiak, Ryszard; "Modeling study of non-line-narrowed hole-burned spectra in weakly coupled dimers and multi-chromophoric molecular assemblies". Chem. Phys., 367, 27-35 (2010).
- Reppert, Mike; Naibo, Virginia; Jankowiak, Ryszard; "Analytical formulas for low-fluence non-line-narrowed hole-burned spectra in an excitonically coupled dimer". J. Chem. Phys., 131, 234104 (2009).
- Reppert, Mike; Zazubovich, Valter; Dang, Nhan C.; Seibert, Michael; Jankowiak, Ryszard; "Low-Energy Chlorophyll States in the CP43 Antenna Protein Complex: Simulation of Various Optical Spectra. II". J. Phys. Chem. B, 112, 9934-9947 (2008).
- Dang, Nhan C.; Zazubovich, Valter; Reppert, Mike; Neupane, Bhanu; Picorel, Rafael; Seibert, Michael; Jankowiak, Ryszard; "The CP43 Proximal Antenna Complex of Higher Plant Photosystem II Revisited: Modeling and Hole Burning Study. I". J. Phys. Chem. B, 112, 9921-9933 (2008).
- Dang, N. C.; Reinot, T.; Reppert, M.; Jankowiak, R.; "Temperature Dependence of Hole Growth Kinetics in Aluminum-Phthalocyanine-Tetrasulfonate in Hyperquenched Glassy Water". J. Phys. Chem. B, 111, 1582-1589 (2007).